Position Statement and Practice Guidance: The link between adult-onset hearing loss and dementia
British Academy of Audiology, British Society of Audiology and The British Society of Hearing Aid Audiologists Joint Document
The UK’s leading hearing loss organisations have joined forces to highlight misleading reports by some health professionals and the media that hearing loss causes dementia, and treating hearing loss will reduce our individual risk of dementia.
In a position statement published in November 2024, British Society of Audiology, the British Academy of Audiology and the British Society of Hearing Aid Audiologists say the misinformation is promoting a sense of alarm and stigma around hearing loss, and may discourage people experiencing hearing difficulties from seeking help.
They also argue the focus on what causes the co-occurrence of hearing loss and dementia could inadvertently distract from much needed research on how to assess and help people who live with both conditions.
The statement published by the organisations, provides a more balanced view of the link between the two, arguing there is no evidence to support or refute either of the claims.
Factors which are predictive of dementia include depression, traumatic brain injury, diabetes, lower levels of education, and social isolation. Hearing loss comes much further down the ranking and has a clear but weak association.
This document presents a joint position statement by the British Society of Audiology (BSA), British Academy of Audiology (BAA) and British Society of Hearing Aid Audiologists (BSHAA). To the best knowledge of BSA, BAA and BSHAA, the position statement represents the evidence-base for the association between adult-onset hearing loss and dementia.
Although care has been taken in preparing this information, the BSA, BAA and BSHAA do not and cannot guarantee the interpretation and application of it. The BSA cannot be held responsible for any errors or omissions, and the BSA accepts no liability whatsoever for any loss or damage howsoever arising. This document stands until superseded or withdrawn by the BSA.
While some areas of cognition improve with age (e.g. knowledge), everyone experiences age-related declines in other aspects of cognition throughout adulthood. Areas of decline include aspects of short term memory, our speed of processing and our ability to process one set of information over another (i.e., inhibition). These changes will affect an individual’s ability to understand speech in challenging environments, e.g. speech in noise, and this is a normal part of ageing. Just because someone experiences age-related cognitive change, and associated auditory deficits, does not mean that they will go on to develop dementia.
Dementia is a group of symptoms that can affect memory, problem-solving, language, and behaviour making it hard for the individual to do everyday activities by themselves. Alzheimer’s disease is the most common type of dementia. Dementia is a major global challenge: the incidence may be decreasing in some high-income countries (Wu et al, 2017), but the number of people living with dementia is growing because of increasing life expectancy. The topic of dementia raises considerable fear and alarm because of the potential devastating consequences for individuals, with a significant impact on families and carers as well as the health and care system.
The well-documented association between adult-onset hearing loss and cognitive decline / dementia (shortened to hearing loss and dementia for ease of reading) is sometimes interpreted as evidence that hearing loss causes dementia, and that treating hearing loss will reduce the risk of dementia. However, there is currently no good evidence to support (or refute) either of these claims. Excessive attention to association and causality may detract from the need for timely clinical research on identification and treatment of people who live with both hearing loss and dementia, and ensuring audiology becomes a dementia-friendly profession.
Clear communication about the hearing loss-dementia link can support realistic expectations and informed decisions. Misleading messages can promote a sense of alarm and stigma around hearing loss and may also discourage help-seeking (Blustein et al, 2023a, 2023b; Dawes and Munro, 2024). There is a need for positive and clear messaging, and this document provides suggestions for the busy practitioner who knows that adult-onset hearing loss can be managed successfully with hearing aids and / or other support. Hearing aids have proven benefits for improving communication with spoken language (Ferguson et al, 2017) and this helps to keep the user cognitively and socially active (Holman et al, 2021, Wells et al, 2020). Therefore, managing adult-onset hearing loss facilitates an active, engaged, independent, and healthy older age (National Academies of Sciences, Engineering and medicine, 2016).
Hearing better can help you to live better; however, it is misleading to imply there is evidence that hearing loss causes dementia, and that treating hearing loss will reduce the risk of dementia.
The aim of this position statement is to present a balanced view on the nature of the association between adult-onset hearing loss and dementia. The objectives are to provide: Hearing better can help you to live better; however, it is misleading to imply there is evidence that hearing loss causes dementia, and that treating hearing loss will reduce the risk of dementia.
- An evidence-based summary on what is known about the nature of the association between hearing loss and dementia as well as the benefit of hearing intervention, with an assessment of the quality of the evidence and its presentation to the public (Section 2).
- Guidance for clinicians, including how to describe the relationship between dementia and hearing loss, including acceptable statements (Sections 3 and 4).
- More detailed definitions of the relevant terminology (Section 5). Those who prefer to receive their information via video, or are less familiar with the relevant terminology, may wish to supplement their reading by viewing a presentation tailored for hearing care professionals by Blustein (2024). This video explains how to communicate clearly and ethically about the link between hearing loss and dementia.
This document is specific to adult-onset hearing loss. As previously highlighted by Blustein (2023), the discussion does not apply to people who identify as being Deaf and are members of a vibrant community that uses sign language to communicate.
Hearing loss is the most prevalent sensory deficit (Mathers, 2000) and the third leading cause of disability in the world (World Health Organization, 2021). Untreated adult-onset hearing loss can result in communication difficulties for spoken language that can lead to social isolation and withdrawal, depression and reduced quality of life (Davis, 2007). Hearing aids with appropriate support are the primary intervention for adults with hearing loss. Hearing aids are effective at improving hearing-specific health related quality of life, general health-related quality of life and listening ability in adults with acquired hearing loss who communicate by speech (Ferguson et al, 2017). The Lancet standing commission on dementia prevention, intervention, and care was published by Livingston et al in 2017 and updated in 2020 and again in 2024. Since prevention is better than cure, the updated report highlights 14 modifiable, and potentially modifiable, risk factors throughout the life course (up from nine in 2017 and 12 in 2020). Hearing loss in mid-life has been identified as a potentially modifiable risk factor for dementia. The updated report also highlights advances in preventative interventions and treatments. The report represents an enormous amount of work and has received widespread attention. However, in its desire to be ambitious about dementia prevention and intervention, identifying hearing loss as a possible modifiable mid-life risk factor has resulted in misunderstandings and misinterpretations on the relationship between hearing loss and dementia. In order to address this, Munro and Dawes (2024) published a commentary on the Lancet commissions specific to the topic of hearing loss. Dawes and Munro (2024) provided a general review of the evidence on the association between hearing loss and dementia, and the benefit of hearing interventions (see Dawes and Munro, 2024). These two articles form the basis of this position statement.
Association between hearing loss and dementia
There is consistent evidence of an association between hearing loss and cognitive decline in adults with acquired hearing loss, dating back almost 150 years (Galton, 1883). Around 30 years ago, Lindenberger and Bates (1994, 1997) reported that age-related declines in hearing (and vision) closely followed declines in cognition. Interest in the connection between sensory function and cognition was given a further boost when Lin et al (2011) reported that baseline levels of hearing loss were associated with incident dementia i.e., new cases of dementia.
Observational studies have shown that people with greater adult-onset hearing loss are likely to have greater cognitive decline. However, association is not causation. For example, the sales of ice cream and sunglasses show a positive correlation (i.e., as the sales of ice cream increase so does the sales of sunglasses) but this is likely due to a common cause (i.e., the arrival of summer weather). This is not to deny the possibility that adult-onset hearing loss may cause dementia. This could occur due to reduced auditory input or lack of social stimulation (direct or indirect causes, respectively), but this has not been proven. Even if adult-onset hearing loss precedes cognitive decline, this does not rule out a common cause. For example, hearing loss could be a marker of dementia because they both share the same underlying cause e.g., vascular disease. Also, hearing loss may be an early manifestation of dementia rather than hearing loss accelerating dementia (Abidin et al, 2021). There is currently a lack of good quality evidence to settle this question.
Association is not causation. It is a mistake to think that if two things co-occur, one must have caused the other.
Do hearing interventions reduce dementia risk?
The possibility of reducing cognitive decline and mitigating dementia risk has been examined in several observational studies, which compared cognitive outcomes for hearing aid users and non-users. Dawes and Völter (2023) reviewed the evidence for both cochlear implants (CI) and acoustic hearing aids. The CI studies report positive outcomes for cognitive improvements, although design limitations of the CI studies make it impossible to attribute these positive outcomes to CIs (rather than to practice effects with repeated cognitive testing, for example). For acoustic hearing aids, the balance of evidence is equivocal: eight studies report positive benefits of using hearing aids and eight did not. More recently, two largescale observational studies have been published. One showed no association between hearing aid use and cognitive impairment (Grenier et al, 2024). The other, by Jiang et al (2023), was retracted when an error in the analysis was discovered (Retraction Watch, 2024): the codes for hearing aid users and nonusers was reversed suggesting risk of dementia was higher (not lower, as originally reported) in hearing aid users compared to nonusers.
A challenge for observational studies is the lack of randomisation to intervention and control groups, which means the results may be biased. For example, typical hearing aid users are better educated than non-users (Sawyer et al, 2019). Education is a factor which reduces the risk of cognitive decline and dementia (Livingston et al, 2024) irrespective of any potential benefit from hearing aids. This makes it difficult to rule out alternative explanations for the potential positive cognitive outcomes associated with hearing aid use. Because of such problems, randomised controlled trials (RCTs) are considered the gold standard in terms of evidence. RCTs involve randomly allocating people to treatment (i.e. hearing aid) or a control group so they avoid any problems with pre-existing differences between users and non-users. Although dementia prevention is the primary interest, having dementia as the main outcome would require an unfeasibly large and long running trial. For practical reasons, trials tend to focus on cognitive change as the primary outcome of interest.
There is no convincing evidence that hearing interventions reduce the risk of dementia in the general population.
The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) RCT investigated use of hearing aids as part of a comprehensive hearing intervention programme, on cognitive decline among a group of people with adult-onset hearing loss (Lin et al, 2023). The main finding of ACHIEVE was negative; i.e., after three years of using hearing aids there was no slowing of cognitive decline. Rates of cognitive decline were the same Association is not causation. It is a mistake to think that if two things co-occur, one must have caused the other. Rates of cognitive decline were the same in the control group of non-hearing aid users as in the intervention group. As such, there is no convincing evidence that hearing interventions reduce the risk of dementia in the general population. A secondary analysis of ACHIEVE reported a benefit of hearing aids in reducing cognitive decline among a subgroup of people characterised as ‘high-risk’ group, but because this was a secondary finding where study-wise error rate was not controlled, this apparent benefit for a subgroup may not be reliable. Further, the benefit reported for the high-risk group was very small and may not be clinically meaningful to the individual (Dawes and Munro, 2024).
Examples of suggested statements, and some statements to avoid, when assessing and managing hearing loss in adults, are provided in Table 1.
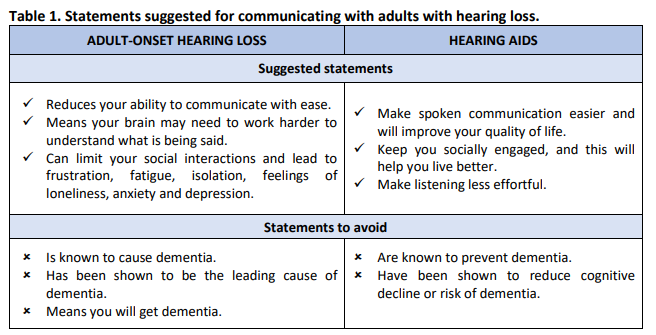
Based on the available evidence, it is our view that:
- Clinicians should fairly represent any association between dementia and hearing loss, based on the available evidence.
- Clinicians must take care not to cause any unnecessary fear or anxiety for their service users due to concerns about hearing loss and dementia.
- Clinicians must never use any association between hearing loss and dementia to encourage or cajole patients to use or acquire hearing aids but should discuss the benefits and problems of hearing aids in a balanced manner. This should be regarded as an issue of professional ethics.
- Marketing materials for hearing devices and hearing services must not indicate any causative association between hearing loss and dementia and must not promote hearing aids as a prevention for dementia. There is no convincing evidence that hearing interventions reduce the risk of dementia in the general population.
- Educators and trainers, in academia and in clinical practice, should highlight the evidence base
and promote professional values to students and trainees.
Suggested responses to Frequently Asked Questions (FAQs) are provided in Table 2.
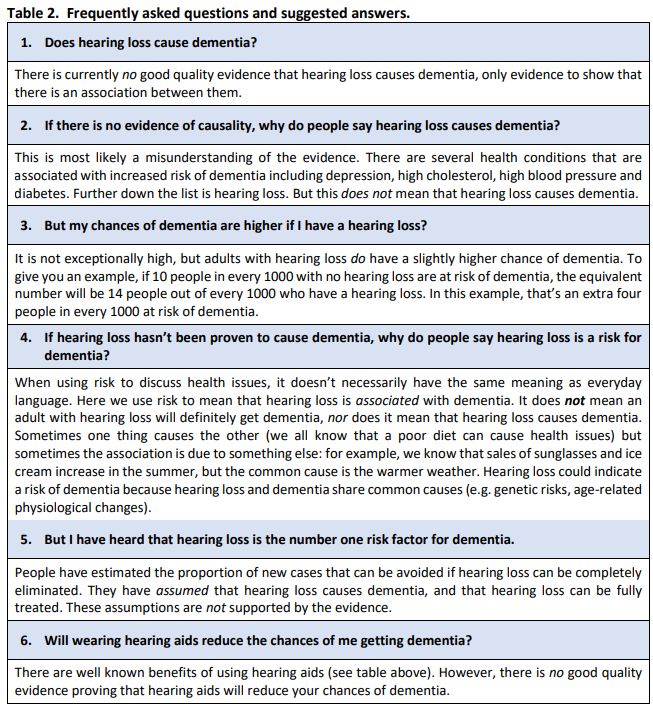

Risk factor: In everyday language, risk is frequently used synonymously with cause. For example, “the heavy rain is a risk factor for flooding” is interpreted as heavy rain will cause flooding if it doesn’t stop. However, in epidemiological studies, risk means an association (or probability), not necessarily a cause. This is an important distinction. Some risk factors are known to modify the chances of disease. It is not appropriate to refer to hearing loss as a “modifiable risk factor” for dementia because it has not been proven that hearing loss causes dementia.
Based on current evidence, we can say that hearing loss is associated with an increased risk of developing dementia. The additional risk is small. However, there is no good evidence that hearing loss causes dementia.
Relative risk (RR): Personal risk to an individual is usually reported as a relative risk. This is the probability of someone with hearing loss developing dementia compared to someone without hearing loss. For example, a RR of 1.4 means the risk of dementia to the individual with the risk factor is 40% higher than someone without the risk factor. For example, if 10 in 1000 people are at risk of dementia, this increases to 14 in 1000 for people with the risk factor. Hearing loss is not the leading personal risk factor for dementia. This is an important point that has frequently been misunderstood. According to the 2024 Lancet report on dementia prevention, intervention and care, the top six personal risk factors for dementia are: depression (RR = 2.2), traumatic brain injury (RR = 1.7), diabetes (RR = 1.7), less education (RR = 1.6), social isolation (RR = 1.6) and untreated vision loss (RR = 1.5). The personal risk of dementia associated with hearing loss is similar to the risk associated with obesity, high levels of low-density lipoprotein (LDL) cholesterol (i.e., the bad cholesterol that can build up in your arteries), and smoking (RR = 1.3-1.4).
Although hearing loss is associated with increased risk of dementia at a population level, it is not a leading risk factor for dementia at an individual level.
Population attributable fraction (PAF): The maximum proportion of new cases of dementia in the population that can theoretically be avoided if the cause can be completely eliminated. PAF is highest for high LDL cholesterol and hearing loss, each 7%. The proportion is high for hearing loss because it is a common condition, despite a relatively low RR. PAF may vary over time and may be different in sub-groups within the population; minority and lower socioeconomic groups often have a higher burden of modifiable risk factors. PAF is concerned with whole populations and is not the same as the personal risk to an individual with hearing loss developing dementia.
The estimated prevalence increased from 31.7% reported in the 2017 and 2020 Lancet commission reports to 59% in the 2024 report. The report did not provide an explanation for the increase in the prevalence estimate. Irrespective of the calculation of PAF, its use remains problematic for the interpretation of dementia risk associated with hearing loss because it assumes, incorrectly, that hearing loss has been proven to cause dementia and that all cases of hearing loss can be completely avoided or entirely mitigated with hearing intervention. The latter is improbable because only a subgroup of people with hearing loss use hearing interventions, even then, hearing aids or cochlear implants do not restore hearing to normal. Finally, some risk factors may co-occur, or lead to a different outcome.
Public facing documents and websites that use the Population Attributable Fraction (PAF) as the basis for claiming that hearing loss is the single greatest risk factor for dementia can be misleading. This is because the public generally assume: (i) this is the personal risk, and (ii) risk means cause.
Abidin FNZ et al (2021). Hearing difficulty is linked to Alzheimer’s disease by common genetic vulnerability, not shared genetic architecture. Aging and Mechanisms of Disease 7.1 (2021): 17.
Blustein J et al (2023a). Messaging clearly and effectively about hearing loss and increased dementia risk. JAMA Otolaryngology–Head & Neck Surgery 149.10, 862-863.
Blustein J et al (2023b) It is time to change our message about hearing loss and dementia. Journal of the American Geriatrics Society 71.8, 2676-2679.
Blustein J. (2024). Communicating clearly and ethically about the link between hearing loss and dementia. Keynote address to the American Doctors of Audiology 2024 Annual Meeting. https://www.audiologist.org/library/topics/conference-videos/item/in-tune-communicating-clearlyand-ethically-about-hearing-loss-and-cognitive-decline-dementia
Davis A. et al (2007). Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models. Health Technology Assessment, 11, 1–294.
Dawes P, Munro KJ. (2024). Hearing Loss and Dementia: Where to From Here? Ear and Hearing, 529-536. doi: 10.1097/aud.0000000000001494
Dawes P, Völter C. (2023). Do hearing loss interventions prevent dementia? Zeitschrift für Gerontologie und Geriatrie, 1-7.
Ferguson M et al (2017). Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev, 9.
Galton F. (1883). Inquiries into Human Faculty and Its Development. London: Macmillan.
Grenier B et al (2024). Hearing Loss, Hearing Aids, and Cognition. JAMA Network Open 7.10, e2436723-e2436723. doi:10.1001/jamanetworkopen.2024.36723
Holman J et al (2021). Hearing aids reduce daily-life fatigue and increase social activity: A longitudinal study. Trends in Hearing, 25, 23312165211052786.
Jiang F et al (2023). Association between hearing aid use and all-cause and cause-specific dementia: an analysis of the UK Biobank cohort. Lancet Public Health. 13: S2468-2667. doi.org/10.1016/S2468-2667(23)00048-8
Lin FR et al (2011). Hearing loss and cognition among older adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 66, 1131-1136.
Lin FR et al (2023). Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. The Lancet, 402, 786-797.
Lindenberger U, Baltes PB. (1994). Sensory functioning and intelligence in old age: a strong connection. Psychology and Aging, 9, 339.
Lindenberger U, Baltes PB. (1997). Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging 12.3, 410.
Livingston G et al (2017). The lancet international commission on dementia prevention and care.” The Lancet 390.10113, 2673-2734.
Livingston G et al (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396.10248, 413-446.
Livingston G et al (2024). Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. The Lancet 404.10452, 572-628
Mathers C et al (2000). Global burden of hearing loss in the year 2000. Global Burden of Disease, 18: 1–30.
Munro KJ, Dawes P. (2024) Commentary: dementia, hearing loss, and the danger of professional rabbit holes. ENT & Audiology News, 27 September. https://www.entandaudiologynews.com/features/audiology-features/post/commentary-dementiahearing-loss-and-the-danger-of-professional-rabbit-holes (accessed 17 November 2024).
National Academies of Sciences, Engineering, and Medicine. (2016). Hearing Health Care for Adults: Priorities for Improving Access and Affordability. Washington, DC: The National Academies Press. https://doi.org/10.17226/23446.
Retraction Watch. (2024). ‘We should have followed up’: Lancet journal retracts article on hearing aids and dementia after prodding. Retrieved from https://retractionwatch.com/2024/01/04/we-should-havefollowed-up-lancet-journal-retracts-article-on-hearing-aids-and-dementia-after-prodding/
Sawyer C et al (2019). Correlates of Hearing Aid Use in UK Adults: Self-Reported Hearing Difficulties, Social Participation, Living Situation, Health, and Demographics. Ear and Hearing 40, 1061-1068, doi:10.1097/aud.0000000000000695
Wells TS et al (2020). Characteristics and health outcomes associated with hearing loss and hearing aid use among older adults. Journal of Aging and Health, 32(7-8), 724-734.
World Health Organization. (2021). World report on hearing. Geneva: World Health Organization.
Wu et al (2017). The changing prevalence and incidence of dementia over time—current evidence. Nature Reviews Neurology 13.6, 327-339.
Produced by: BSA Professional Guidance Group
Key Authors:
| Name | Organisation |
| Kevin J Munro | University of Manchester; Manchester NHS Foundation Trust, England. |
| Claire Benton | Vice President, British Academy of Audiology; Audiologists, Skipton, England. |
| Jan Blustein | New York University, New York, NY, USA |
| Piers Dawes | University of Queensland, Australia. |
| Helen Henshaw | Chair, Cognition in Hearing Special Interest Group, British Society of Audiology, NIHR Nottingham Biomedical Research Centre; University of Nottingham, England. |
| Shahad Howe | Co-Chair Adult Rehabilitation Interest Group, British Society of Audiology; North East Hearing and Balance, Darlington, England; Consumer Engagement Manager, Advanced Bionics, UK. |
| Linor Jones | Co-Chair, Professional Guidance Group, British Society of Audiology; Betsi Cadwaladr University Health Board, Wales. |
| Ted Leverton | Service users and volunteer for RNID; Bere Alston, Devon, England. |
| Laura Turton | Board Director, British Academy of Audiology; NHS Tayside, Scotland. |
| Aparna Mordekar | Sheffield Health and Social Care NHS Foundation Trust, England. |
| Michael Marchant | Vice President, British Society of Hearing Aid Audiologists |
| Richard Windle | Vice Chair, British Society of Audiology; Kingston and Richmond NHS Foundation Trust, England. |
| Tracy Pinkerton | Northern Health and Social Care Trust, Northern Ireland |
The authors represent professionals in in the field of hearing loss (including the UK Devolved Administrations) and old age psychiatry, service users and BSA, BAA and BSHAA.
Declarations of interests
SH is employed as a Consumer Engagement Manager with Advanced Bionics, UK. HH reports speaker honoraria and travel expenses from GN Hearing A/S paid to the University of Nottingham.
Citation
Please cite this document in the following format:
British Society of Audiology, British Academy of Audiology, The British Society of Hearing Aid Audiologists (2024), The link between adult-onset hearing loss and dementia. [Online]. Available from: insert web link. [Accessed date]
