Starting from zero - A first steps in quality management toolkit
1. Starting from zero
This guide and its accompanying resources are here to help support you in starting a quality improvement cycle.
Starting a quality cycle can be quite intimidating. There are many different aspects, all interlinked… should you start by trying an audit? Setting up non-conformance processes?
This guide considers documentation as a starting point and builds from there. This may work for your service’s needs, or it may not. Either way, hopefully this will provide something useful to help in your quality improvement cycle.
2. Definition & documentation
One of the most important foundations of a quality cycle is documentation. It is from documentation you can establish processes to audit and check non-conformances against.
However, before starting to create documentation we need a document control process, and before THAT we need to consider how we define the process and the documents it controls.
Having a clearly defined document control process will not only make it easier to produce and manage documentation, but also give staff confidence in the process – something vital in establishing an ongoing (and robust) quality improvement cycle.
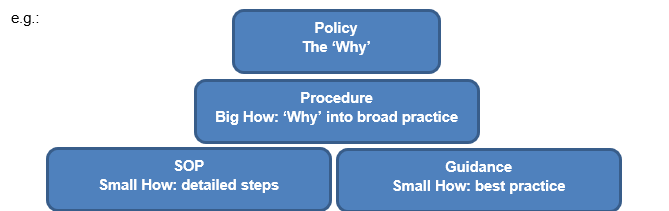
2.1. Defining document types
One of the first things you will need to define are the types of documents you are going to use within the department. It’s important to make sure that your definition of a document type also fits with the common understanding of that term, so don’t be afraid to take some time to research the types and their terminology. This is also a process which can be added to later, so it’s ok to start simple and build from there.
Wherever possible try and keep your definitions succinct and easy to understand e.g.
“Policy – this is the why of all other documentation”
Or
“SOP – step by step points to complete a standardised task”
You may also find it useful to consider your document hierarchy – this is something which can come in useful later when developing future document plans.
2.2. Defining responsibilities
With your definitions coming together you will need to consider your responsibilities in the document control process
- Who can write a document?
- Who can authorise a document?
- Who is responsible for formatting the document?
- Who is responsible for issuing the document?
- Who is responsible for monitoring readership?
- Who is responsible for retiring and archiving previous versions?
Perhaps you have quality champions or a quality group that can oversee this? Or perhaps you need to assign the responsibility? In the early stages your document control process may go through numerous revisions, so a group which only meets quarterly may not be the best choice!
2.3. Defining readership groups
Depending on the size and specialisations within your service you may need to consider the readership groups you have for each document e.g. if you see both Adult and paediatric patients you may need to define the groups as ‘Adults clinical staff’, ‘Paediatrics clinical staff’ or ‘all clinical staff’.
This helps ensure that staff only read what they need to & that authors focus on the correct staff groups when creating documents.
Thinking about these groups at the start will also prove useful later when you start to implement audit and non-conformance processes.
2.4. Defining timeframes
A key aspect of document control is the timeframe for review i.e. how long will your documents last until review? The general consensus is that documents should be reviewed within 3-4 years to ensure they are current to accepted practice.
There may some documents that require shorter review windows – Quality polices and Quality manuals should be reviewed yearly to ensure they match current quality objectives.
Other documents may be linked to non-clinical processes with little change, so could have a longer review window.
Whatever you decide on, make sure it is clearly defined in your document control procedure.
You will also need to consider when your review process is due to start. The document list included in this pack highlights documents 6 months before expiry for their review start – the hope being that the review is completed in this time resulting in a seamless move between versions. However, you may find that at the start the review process takes longer so you need to start sooner.
2.5. Defining the review process
Eventually, your documentation will need review. Hopefully this will be at the end of its review date, however it can happen sooner depending on process changes or new practice.
Having a defined process for review will help avoid confusion on what is expected of a document review i.e. do you expect a full re-write? Or can the document stay as is with only minor changes where applicable? Should all the references be checked by the reviewer or party responsible for document control? Who notifies the author that the document is due review? And who monitors the review process to ensure it’s completed in time?
It’s also worth considering how the review will be reflected in staff readership e.g. if a document hasn’t been changed following review – simply renewed, do you expect your staff to read it again to refresh your knowledge?
If part of your document has changed, are you expecting your staff to re-read the whole document or just the parts which have changed? If it’s the latter, you will need to make sure your use of the ‘changes from previous version’ aspect of a document is correct and easy to understand.
2.6. Check what is already in place
With document control, and all quality processes, it is important to see what your Trust already has in place. It may be that the Trust wants all local documentation to be subject to the same controls as Trust wide documents – this is a factor which could add to things like review timeframes, however it could also give an avenue for extra support.
2.7. Creating a template
An essential step in starting your document control process is having a standard document template (one is included in this resource pack). Having this ensures standardisation through your documentation at the start and compliance to current accreditation requirements.
If you chose to adjust your template at a later date, you will also need to consider if all your documentation will need updating to the new format or if you will update at point of review. If you choose the latter, ensure this is documented somewhere along with the rationale as it may be challenged.
Large scale changes to document templates can be quite time consuming if you have lots of documents, so take the time to ensure the template is sufficient to your needs.
2.8. Managing documents
As your documents come together you will need to consider how to manage them. If you have a designated individual or team who is responsible for document control this can be one of their tasks.
It is highly recommended you consolidate all documentation into one shared drive location & order the documents according to type or speciality. You will also need to check shared drive locations every now and then to ensure there is no proliferation of outdated or draft versions.
It can be very tempting to create a draft document in a specialisms area of a shared drive, however this makes it harder to monitor. So in addition to having all current documentation in one location, it can be useful to have a designated space for draft documents too.
When documents are retired or superseded you will need to retain copies to support in non-conformance and audit investigations. It can be useful to keep these versions on areas of the shared drive with limited access – this will again help prevent staff from reading incorrect versions.
As part of your contingency planning you may also wish to retain hard copies of documentation in the event of a catastrophic IT fault.
2.9. Monitoring readership
As soon as you start to issue out documentation you will need to devise a way of recording document readership. This is a really important part of the process, as it forms part of the assurance that staff are competent in their role.
A simple way to do this is to create a spread sheet with all your current documentation and each staff members name with a blank cell for them to add a date when it has been read.
This process will again support in future audit and non-conformance processes.
2.10. Which documents first?
With the above in mind, your first document may well be your document control procedure. You may also wish to create a quality policy at this stage as it will help inform the development of your quality cycle going forwards. Quality polices do not need to be particularly long & many examples can be found on the internet for inspiration.
With those two bits of foundation in place you can then start to consider your clinical documentation. A good way to prioritise is to think in terms of the Pareto principle/ 80-20 rule. In this situation, the idea would be that 80% of your clinical work comes from 20% of your clinical processes. Due to the volumes involved, there is a likelihood these will also contain the largest number of non-conformances, so defining them first will help to ensure conformance to standards (not to mention making it easier for new starters to learn your most common processes!).
It can be very tempting to start producing lots of documents very quickly, however, you may soon find your staff base overloaded with documents to read and some very painful document review months later on.
To combat this, it is suggested you create a document plan. Here you can lay out your goal for your documentation and also see how documents can inform each other and be interlinked – you may also find opportunities to consolidate documents under one banner. An example of this is having multiple documents relating to the management of staff (i.e. Annual leave processes, dress code etc) where they can be rolled into one ‘Staff management’ document.
Don’t be afraid to stagger the launch of your documents and set limits to what you realistically expect your staff to read in a month.
An example of a document control procedure is included within this resource pack.
2.11. Quality policy example
Needs of users
In order to meet the needs and requirements of its users the Audiology service will:
- Look to consult with users on a regular basis to ensure their needs and requirements are met.
- Set quality objectives and plan to implement and maintain this quality policy.
- Ensure that all personnel are familiar with the quality policy to ensure user’s satisfaction.
- Operate a quality management system that integrates qualitative metrics indicative of its performance, documents its procedures and keeps records that provide evidence of the proper conduct of its activities. Where this cannot be supported by the implementation of an ICT based QMS, the service will develop its own in house QMS based process.
- Commit to providing facilities required for the provision of the service and for the health, safety and welfare of its staff.
- Ensure that visitors to the service will be treated with respect and that due consideration will be given to their safety whilst on any site where Audiology operates.
- Comply with local and national environmental legislation and guidelines.
- Uphold professional values and be committed to good professional practice and conduct.
Compliance with National, Local and regulatory standards
To comply with the above standards, both compulsory and aspirational, the service is committed to:
- Recruitment, training, development and retention of staff at all levels in a manner that ensures that they are competent to be fully involved in its work.
- Maintenance, management and procurement of equipment, facilities and other resources as are needed for the provision of the service and ensure the quality of its examination results.
- Use of evidence based procedures that will ensure the highest achievable quality of all examinations performed.
- Undertake regular internal quality monitoring activities to ensure compliance with standards.
- Be subject to external audits to ensure compliance with standards.
- Reporting results of appointments in a manner which ensures their timeliness, accuracy, and clinical usefulness and maintains confidentiality.
- Evaluation and continual improvement of the service it provides to its users.
- Maintain a group of staff (comprised of clinical and non-clinical staff members) to review, monitor and advise on quality related matters throughout the service.
3. Evaluating and evolving processes
As you start defining and documenting your processes you are now in a place to start the next steps of evaluating and improving upon them. These steps have two main components – Audit and Non-conformance/CAPA.
With staff now familiarising themselves with standardised documented processes you can start to build assurance that they are doing what they are supposed to!
If you try to implement these processes without defined and documenting what you do, you will likely find that a lack of documented & defined processes sits high up in your RCA’s.
3.1. Audit
An example audit procedure is included in this resource pack, it is titled ‘Procedure – Quality Monitoring’.
3.1.1. Starting/Creating audits
Audit can be quite a vast and complex subject – even more so if you’re starting from scratch. Like all quality processes it can be good to start small and build upon it. With that in mind, rather than starting with a full service review you may find it easier to start with monthly quality assurance audits.
The audit tool included in this resource pack is designed to take a lot of the pain out of getting such a process established. Its function is to support an audit of compliance, simply put: questions which can have yes or no answers.
Developing questions can be a bit tricky, so it’s good to think about what some of the standard actions in an appointment might be – taking consent, checking equipment, producing reports etc. and combine that with key actions in your documentation.
The worked example of the audit sheet shows an audit for assessments for Bone anchored hearing, it has 11 questions. These questions were developed through looking at standard actions across all specialisms and those specific to this type of appointment.
When you first create and run a compliance audit you may find that some questions didn’t work quite as well as expected. With this in mind, you may wish to have a defined ‘trial’ period for any new audit, after which the questions should be ‘set’ until the end of the audit cycle.
It can be very tempting to keep changing questions throughout the audit, however doing that you start to lose the validity and integrity of your process, so having a trial period can be a good way around this.
Compliance audits are fairly simple to implement and easier for a wider staff base to pick up and build confidence with. Someone new to audit may get nervous if you start to talk about them doing a vertical audit, but say you’re doing an audit where you answer yes or no – well, it keeps things simple and helps build towards bigger things.
3.1.2. Sample sizes
As your audit’s start to develop you will also need to think about how you work your sample sizes. One of the key things about doing audits is to give them some form of statistical validity.
There are several tools available on the internet to help you figure out what your sample size should be. This kind of thing can be quite intimidating at first so please make sure you talk to audit leads in your Trust, or the wider NHS space, to get help in understanding it.
You may wish to spread your sample size across the year and do part of it each month.
3.1.3. Audit ratings
As part of your audit set up you will need to consider what you consider an acceptable result. This may change between audits or even between questions on the audit.
Ideally, we would be looking at SPC charts to review our findings but that takes a lot of work to set up, so for ease you may prefer to use a RAG rating.
The audit tool included in this resource pack has the following rating set up:

If you change the RAG rating on the ‘year’ tab it will change the rating throughout the sheet.
You may wish to run a few audits to get a sense of what your current status is to inform this rating.
3.1.4. Audit schedule
As your audits start to take shape you will need to create an audit schedule. The resources in this pack includes a simple audit template which can calculate if your audits are on schedule or not.
Part of this is to consider what classes as a completed audit: Will your individual auditors record when they consider themselves done? Or will it be when their findings are shared? Will you have set individuals to record this information? Considering this at the start will help keep your process clear and concise later on.
If you are part of a team with multiple parts (e.g. Adults, paediatrics, vestibular), you will need to agree the timeframe for your audit cycle – are you following calendar or financial year? If you all adopt the same approach it can be much easier to look at commonalities and challenges across the wider service – as well as allowing for greater co-ordination at later steps in accreditation (such as annual service performance review meetings).
As part of your review cycle you should also consider a ‘review’ period where audit questions and the audits themselves are checked for suitability going into the new year. This is also a good time to introduce any new audits.
3.1.5. Audit findings
As part of your audit programme you will need to consider how and when your audit results are shared with the wider team.
The audit tool included in this resource pack has an export function where it will create a report for the current month against the current average result for the year. This can be sent via email or key information taken from it for sharing however is preferred.
As part of the sharing it’s good to highlight what parts of the audit are performing well as well as spots for improvement.
As part of your auditing you may find new processes or paperwork being introduced as a response to audit findings. These changes should be noted against the audit so you can monitor their influence. The audit tool contains a tab for ‘process updates’ where you can record this.
3.1.6. Escalation of results
As your audit process is established you will need to consider what constitutes poor performance and how it is escalated. Is a one off red rating cause for concern? Is the concern dictated by how ‘red’ it is? Are you looking for a run of poor results before action? If criteria for escalation are met, what is the escalation route?- escalation to senior colleagues or quality leads or raising CAPA’s?
Getting an idea of these factors early on will help your process to become robust and easier to follow for those doing the auditing – not to mention making it easier for accrediting bodies to follow.
3.2. Non-conformance/CAPA
When a process isn’t followed, or needs action to avoid non-conformance you need a way to record, investigate and follow up on it. This is typically a Non-conformance or CAPA process. For simplicity, this guide will use the term CAPA going forwards.
- What is a non-conformance? A non-conformance is when there is a failure to meet specified requirements (e.g. a standardised process is not followed correctly!)
- What is CAPA? CAPA incorporates non-conformance along with preventative actions:
- CA: Corrective actions
- PA: Preventative actions
To help in understanding the CAPA process a copy of a CAPA procedure is included in these resources. What follows are some of the considerations to take when setting up a CAPA system.
3.2.1.Trust systems
Your Trust will likely already have its own incident reporting system. Before starting you CAPA process it is really important to understand what your Trusts system is designed to cover and where the gaps are your CAPA system can fill – e.g. there may be less concern at a Trust level that a form was not completed correctly but it could cause issues at a local level.
Having a clear line where your CAPA system ends and Trust systems begin is very important – not only for creating the process but also making it clear to staff what systems they should use.
3.2.2. Reviewing CAPA’s
As part of establishing a CAPA process you will need to decide who will actually review the CAPA’s. Will you have a cross service team or nominated individuals? Will you rotate who does it?
Reviewing submissions and creating action plans can be quite time consuming, so you need to be realistic in what your respective team size can achieve. You may wish to combine these factors with the rating of each CAPA to determine how you manage your action planning.
3.2.3. Carrying out corrective actions & Shared learning
One of the most important parts of the CAPA system is sharing learning. This is particularly important if you start to find commonalities or repeat offences when reviewing. If you are starting from zero staff may not be accustomed to this, so it’s really important it’s presented to your team as helping to improve rather than being penalised. One way to think on it is if you had ink on your face surely you’d want someone to tell you? That is the best way to view and utilise this system. It being seen as a way to ‘dob people in’ will not encourage positive participation.
More information on CAPA can be found in the accompanying procedure and guide.
4. Informed improvement
As your audit and CAPA processes start to become established you may start to find points for improvement in your documentation that you originally hadn’t considered. However, you will now hopefully be in a much more informed position to make changes.
It is important that you look to collate some of these improvements (these are good things to note within your document plan) and resist the urge to keep constantly changing processes, whilst its very tempting you will start to find change fatigue taking place within your team.
5. Next steps
With your document control, Audit and CAPA processes starting you are in a good place to start thinking about creating a quality manual.
A quality manual is a key component of current accreditation requirements. This can be quite a large and encompassing document! However, there are an increasing number of Audiology quality manuals being created that you can use for reference before creating your own (one of which is included in this resource pack).
A Quality manual is quite simply a manual for all the quality systems you have in place in your department. Some of these will be local and others will be Trust wide. A quality manual may seem like a heft undertaking, but it can provide benefits in sharing understanding of your service beyond just your own staff.
6. The road to accreditation
Hopefully, this guide will support you on your first steps towards accreditation by establishing systems which will support further growth of your quality processes.
Make no mistake, there is quite a lot involved in accreditation! But hopefully you will now have a starting point. Quality improvement can seem a bit like a proverbial snake eating its own tail – this is the first bite
Read the following documents first as you start from zero:
Audiology Quality Manual Procedure
Then work through the sections below:
